Biotech
University of Barcelona Unveils Breakthrough Strategy for Brain Regeneration
A University of Barcelona study introduces a dual strategy for brain repair by combining stem cell therapy with sustained BDNF delivery. Using genetically modified human neural progenitor cells, researchers achieved improved neuronal maturation, connectivity, and chemoattraction. This approach may enhance brain regeneration, offering promise for treating neurodegenerative diseases and brain injuries.
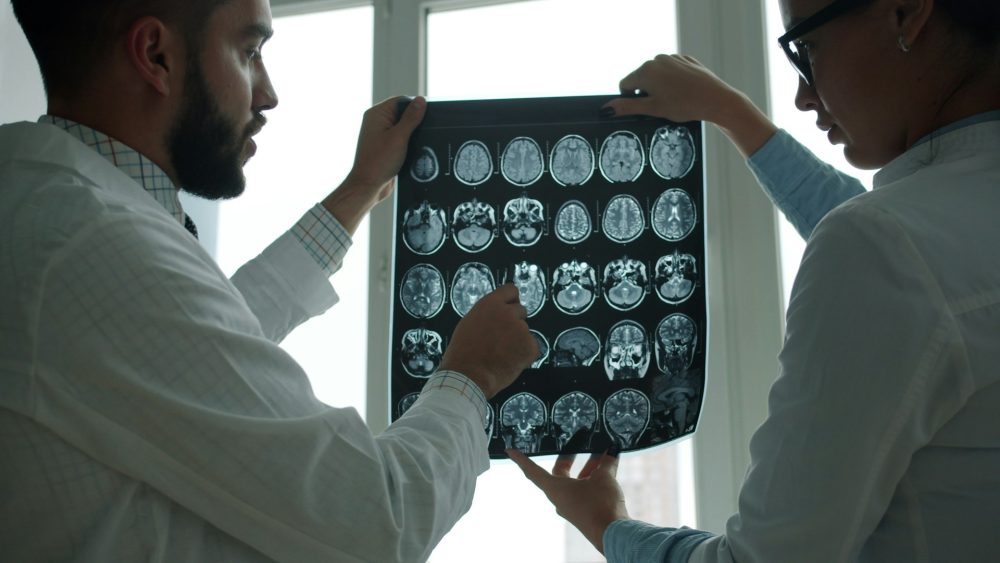
Brain regeneration after injury or neurodegenerative disease remains one of the greatest challenges in modern medicine. Despite advances in neuroscience, the mechanisms of neuronal repair and synaptic plasticity remain poorly understood.
A new study led by the University of Barcelona proposes an innovative strategy based on the combination of cell therapies with the sustained administration of brain-derived neurotrophic factor (BDNF), with the aim of promoting neuronal maturation, functional connectivity, and the integration of transplanted cells into damaged brain tissue.
The work, published in the International Journal of Molecular Sciences, was developed by researchers from the Institute of Neurosciences at the University of Barcelona (UBneuro) and the Faculty of Medicine and Health Sciences. The research proposes an approach that combines cell therapy with genetic bioengineering to enhance central nervous system regeneration.
A dual strategy to repair the brain
Neurodegenerative diseases, such as Alzheimer’s and Parkinson’s, along with traumatic brain injuries and stroke, represent a growing burden on healthcare systems worldwide. These pathologies cause the irreversible loss of neurons and synaptic connections, resulting in cognitive, motor, and behavioral impairment. Since the human brain’s natural ability to regenerate is limited, biomedical research seeks to develop therapies that can replace damaged cells and restore lost neuronal function.
In this context, stem cell therapies have emerged as one of the most promising lines of research. These cells possess the ability to differentiate into different neuronal types and establish new connections, opening up the possibility of regenerating damaged brain circuits. However, one of the main obstacles remains the functional integration of transplanted cells and their long-term survival in the injured brain environment.
The University of Barcelona team addressed this challenge using a strategy that combines the use of induced pluripotent stem cells (iPSCs) —reprogrammed from human skin cells— with the continuous expression of BDNF , a key protein in the development and maintenance of the nervous system. BDNF promotes neuronal survival, stimulates axonal growth, and enhances synaptic plasticity, all of which are essential for the brain’s functional recovery after injury.
Improve neuronal maturation and connectivity
The researchers generated cultures of human neural progenitor cells genetically modified to overexpress BDNF, observing a significant increase in neuronal maturation and synaptic activity. This effect was accompanied by a greater number of mature neurons without altering the organization of neuronal networks , suggesting that the protein stimulates cellular function without inducing structural alterations.
The study also revealed that cells that produce BDNF have the ability to attract the axons of other neurons, a phenomenon known as chemoattraction. This finding was confirmed using microfluidic chips, a technology that allows neuronal populations separated by microchannels to be cultured and how they communicate analyzed. The results showed that axons preferentially gravitate toward regions with higher BDNF concentrations, which could facilitate the integration of neuronal transplants into the recipient brain.
These observations are relevant because the precise orientation and connection of transplanted axons is essential for restoring functional neuronal circuits. The study suggests that the sustained secretion of BDNF by transplanted cells could act as a natural chemical guide, improving connectivity between new and existing neurons in brain tissue.
Although the results are preliminary and obtained in laboratory models, the authors highlight the potential of this strategy for application in neurodegenerative diseases or brain injuries in animal models. Specifically, the research group has been working for years on cell therapies for ischemic stroke, a condition that causes severe damage to the cerebral cortex and leaves persistent motor and cognitive sequelae.
The findings also contribute to overcoming some historical limitations of stem cell therapies. In ongoing clinical trials for Parkinson’s disease, for example, it has been shown that dopaminergic cells derived from human pluripotent stem cells can integrate into patients’ brains and improve motor symptoms. However, efficacy varies, and functional integration is not always achieved. Incorporating neurotrophic factors such as BDNF into the design of these therapies could increase cell survival and the quality of established connections.
The research also suggests that controlled BDNF expression does not interfere with the formation of stable neuronal networks, avoiding the risk of hyperexcitability or disorganized connections. This balance is crucial to ensuring the functionality and safety of neuronal transplant-based therapies.
Future prospects for brain regeneration
The next step will be to validate these results in animal models to determine whether the improvements observed in vitro can be translated into measurable functional benefits , such as motor or cognitive recovery after brain injury. This preclinical phase will be crucial for evaluating the feasibility of using genetically modified neural progenitor cells in cell replacement therapies.
In the long term, the goal is to develop personalized treatments that combine the precision of cellular engineering with the brain’s own reparative capacity. This integrated approach could mark a turning point in the treatment of diseases that currently have no cure and open up new opportunities in neurorehabilitation and regenerative medicine.
Recent advances in gene editing, tissue bioengineering, and translational neuroscience make it possible to imagine a future in which neuronal regeneration ceases to be a theoretical concept and becomes a clinical reality. The combination of human stem cells with controlled BDNF production is emerging as one of the most promising avenues for restoring lost brain function and improving the quality of life for millions of people affected by neurological disorders worldwide.
__
(Featured image by Vitaly Gariev via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in GACETA MEDICA. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Business2 weeks ago
Business2 weeks agoDow Jones Breaks 50,000 as Bull Market Surges Amid Caution and Volatility
-

 Crowdfunding2 days ago
Crowdfunding2 days agoThe Youth Program at Enzian Shooting Club Is Expanding Thanks to Crowdfunding
-

 Impact Investing1 week ago
Impact Investing1 week agoEU Backs 90% Emissions Cut by 2040 and Delays ETS2 Rollout
-
 Markets5 days ago
Markets5 days agoMarkets, Jobs, and Precious Metals Show Volatility Amid Uncertainty













