Biotech
CAR-T Therapies: From Breakthrough Cancer Treatment to Faster, Safer, and More Accessible Immunotherapy
CAR-T therapies have transformed hematologic cancer care, achieving high remission but facing relapse, toxicity, cost, and slow manufacturing. Innovations include mRNA-based in vivo CAR-T generation for speed and reversibility, dual-target CAR-T to prevent antigen escape, ultra-rapid 48-hour production, and public initiatives optimizing safety, durability, and affordability, expanding applications to solid tumors and autoimmune diseases globally.
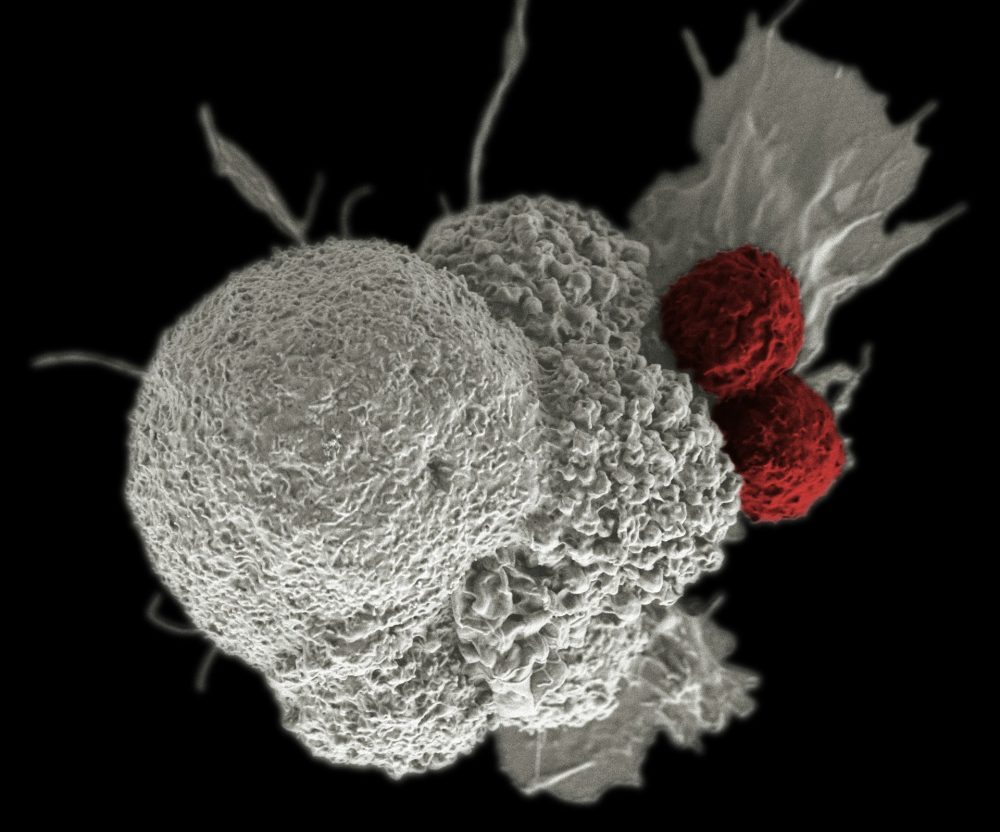
Chimeric antigen receptor (CAR-T) T-cell therapies have moved beyond a futuristic promise to become a therapeutic tool with enormous potential, primarily in the field of hematology-oncology, and are opening new frontiers in solid tumors and autoimmune diseases.
This revolutionary approach involves the genetic modification of a specific type of white blood cell, the T cell, so that it expresses an artificial receptor (CAR) designed to recognize and eliminate specific proteins present on tumor cells.
Although CAR-T cell therapies represent an unprecedented advance in the treatment of refractory hematological malignancies, achieving remission rates of up to 80% in acute lymphoblastic leukemia and exceeding 85% in myeloma, current treatments face critical challenges.
These challenges include the risk of relapse (which occurs in approximately half of cases during the first 18 months), high toxicity, and the costs associated with complex manufacturing, which strain the sustainability of public healthcare systems. Recent research is addressing these problems from multiple angles, from optimizing production to more sophisticated molecular design and personalized treatment.
The mRNA revolution: accelerating the fight
The traditional manufacturing process for CAR-T therapies is one of their main limitations. It requires the collection and modification of the patient’s own immune cells, a complex process that can take weeks or even months. This delay poses a critical clinical challenge , as patients with advanced cancer may die before receiving treatment.
Traditionally, manufacturing CAR T-cell therapies requires specialized facilities where viruses are used to insert the CAR gene into T cells extracted from the patient. In the search for faster and less expensive methods, messenger RNA (mRNA) technology , similar to that used in some COVID-19 vaccines, has emerged as the most promising alternative.
A study published in Science has presented an innovative strategy that stimulates the body to generate its own CAR-T cells in situ . Instead of modifying cells in a laboratory, lipid nanoparticles (tiny capsules composed of lipids) loaded with mRNA encoding the CAR receptor are administered. These nanoparticles were designed to specifically penetrate the T cells.
The results of this strategy in mice were astonishing: in less than three hours , large numbers of CAR T cells appeared in the blood, spleen, and lymph nodes. With just two doses, the tumors shrank rapidly and virtually disappeared within three days in mice carrying human tumors. This approach would not only significantly shorten waiting times to begin treatment but would also help reduce the high costs associated with traditional manufacturing.
The key to safety: reversibility for autoimmune disorders
In addition to speed, mRNA technology offers a crucial advantage in terms of safety, especially for potential use in autoimmune diseases such as multiple sclerosis, lupus, or myasthenia gravis. Unlike viral DNA-based approaches, which induce more lasting cellular modification, mRNA does not integrate into the cellular genome and is rapidly degraded after fulfilling its function.
This feature acts as a natural mechanism for switching off the therapy . In a monkey model, administering nanoparticles with mRNA designed to generate CAR-T cells that eliminate B lymphocytes (cells involved in autoimmune diseases) led to a decrease in the levels of these lymphocytes, a phenomenon that could translate into lasting remissions. The cessation of CAR production after a certain period allows B lymphocytes to regenerate, facilitating the recovery of the immune system, something that would be more difficult with long-lasting DNA modifications.
However, toxicity research must continue, as one of the animal models using this new method developed a life-threatening condition that causes severe inflammation, an effect occasionally seen in patients treated with laboratory CAR-T cells.
Overcoming antigenic escape: the Tandem strategy
One of the main challenges in hematologic oncology, even after initial success with CAR-T cell therapy, is relapse, which persists beyond three years in 35–60% of patients. The primary cause of these relapses is antigen escape , where tumor cells “hide” the target molecule (such as CD19) that standard CAR-T cell therapy detects.
Faced with this problem, the CRIS Unit for Advanced Therapies in Childhood Cancer and the La Paz University Hospital have developed and implemented a pioneering strategy in Europe: CAR-T therapy in Tandem or Dual .
This innovation involves genetically modifying the patient’s T lymphocytes so that they simultaneously recognize two different molecules: CD19 and CD22 . In this way, if the tumor masks one of the targets, the other is detected, significantly hindering the relapse mechanism.
The results of this immunotherapy, offered on a compassionate use basis for children and young adults with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) who had no other treatment options, have been compelling. After an average follow-up of 20 months, eight of the initial 12 patients are alive, disease-free, and enjoying a good quality of life .
In an analysis of high-risk patients, an overall survival rate of approximately 70-72% was observed. In nine of the eleven treated patients, the disease was reduced to undetectable levels one month after the infusion, and six were able to receive a bone marrow transplant to consolidate the treatment. This success has led the CRIS Unit to initiate an official clinical trial to validate and expand the application of this strategy.
The impact of CAR-T therapy in real life is evident in stories like that of Mar, the first patient treated with CAR-T at the Gregorio Marañón Hospital for diffuse large B-cell lymphoma (DLBCL). After six years of treatment with Yescarta (axicabtagene ciloleucel), Mar has maintained complete remission, celebrating two birthdays in June: her own and the anniversary of the day she received the life-changing CAR-T therapy.
Recent studies presented at the 2025 European Hematology Association (EHA) Annual Congress confirm the efficacy of axicabtagene ciloleucel as a second-line treatment for relapsed/refractory DLBCL, showing an overall response rate of 79% and an overall survival rate of 74% at twelve months.
The design of cell survival and sustainability: the Andalusian axis
The development of CAR-T therapy focuses not only on immediate efficacy but also on the durability of the response, safety, reduced toxicity, and economic viability. The Andalusian Public Health System (SSPA) has addressed this challenge through the ambitious CART_ANDALUCÍA Project .
With a budget of €4.28 million, managed by the Andalusian Public Foundation for Progress and Health (FPS), the goal is to develop a “more effective, safer CAR-T therapy with fewer adverse effects .” The project’s scientific strategy focuses on optimizing the “survival and memory mechanisms of T cells” to achieve prolonged immunological persistence.
To increase safety and minimize adverse effects, researchers are investigating “adjustable control systems” that allow for modulation of therapeutic activity. Furthermore, to counteract tumor escape (the main mechanism of relapse due to loss of the target antigen), multispecific or dual CAR-T cells are being developed that can recognize different antigens, thus addressing tumor heterogeneity.
A fundamental pillar of the project is economic sustainability . The framework for action is Public Procurement of Innovation (PPI), a model that allows the public administration to act as a direct driver of R&D. Instead of funding closed projects, PPI presents a challenge to the scientific sector, ensuring that the knowledge and equipment generated remain within the public system, avoiding external dependencies and promoting local production.
The goal is a cost-effective model that guarantees accessibility within the Andalusian public healthcare system. The FPS expects to begin implementing this new solution in the Andalusian Public Health System (SSPA) within approximately five years.
CAR-T in 48 hours: speed and feasibility
Another milestone in production optimization is the development of ultra-fast CAR-T therapies, such as rapcabtagene autoleucel using the T-Charge platform. The traditional limitation of manufacturing is the long cell culture (20 to 25 days) required to expand the CAR-T cell population, which can be too slow for patients with aggressive tumors.
T-Charge technology eliminates the expansion phase , reducing manufacturing time to less than two days (48 hours). This is not only vital for lead time, but also prevents cells from becoming “exhausted” from prolonged cytokine exposure, increasing their potential efficacy.
The results of a phase 1 clinical trial of this innovative therapy, targeting CD19 for relapsed or refractory B-cell acute lymphoblastic leukemia, showed a manageable safety profile and promising antitumor activity. The best overall response ranged from 70% to 100%, depending on the dose. This balance of safety, efficacy, and rapid production opens the door to its integration into routine clinical practice.
__
(Featured image by National Cancer Institute via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in GACETA MEDICA. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Impact Investing2 weeks ago
Impact Investing2 weeks agoEU Backs 90% Emissions Cut by 2040 and Delays ETS2 Rollout
-

 Crypto1 day ago
Crypto1 day agoTariff Turmoil Sends Bitcoin and Ethereum Lower as Crypto Markets Face Mounting Pressure
-
 Markets1 week ago
Markets1 week agoMarkets, Jobs, and Precious Metals Show Volatility Amid Uncertainty
-
 Cannabis5 days ago
Cannabis5 days agoAI Can Mimic Psychedelic Experiences but Cannot Truly Feel Them, Study Warns






















