Biotech
Colon Cancer Micrometastases Identified Through Blood Test
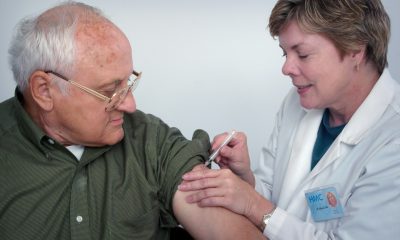
Researchers from INCLIVA developed a blood-based test, TAV16, to detect micrometastases in early-stage colon cancer with unprecedented sensitivity. Using ctDNA analysis, it predicts relapse risk more accurately than current methods. Validated internationally, the test may reduce unnecessary chemotherapy and enable early immunotherapy. The study marks a breakthrough in noninvasive, personalized cancer monitoring and treatment.

A team from the INCLIVA Health Research Institute, affiliated with the University Clinical Hospital of Valencia, has led a pioneering study that improves the detection of micrometastases in colon cancer through a simple blood test. This breakthrough paves the way for the development of new therapeutic strategies, such as the application of immunotherapy in early stages of the disease.
The findings are collected in the study entitled Whole exome tumor-agnostic ctDNA analysis enhances minimal residual disease detection and reveals relapse mechanisms in localized colon cancer, recently published in the scientific journal Nature Cancer.
The study’s main objective was to improve the early detection of relapse in patients with localized colon cancer (stages II and III) using an advanced, noninvasive technique based on blood tests. This tool allows for more accurate prediction of whether a patient undergoing surgery will develop a relapse, even before it is visible through imaging techniques.
To this end, the team developed a proprietary test—called TAV16 —based on whole-exome sequencing of circulating tumor DNA (ctDNA) present in blood samples. This technique was able to detect the presence of minimal residual disease after surgery with unprecedented sensitivity, allowing researchers to predict which patients are at greatest risk of relapse and which could avoid unnecessary additional treatment.
The analysis was conducted at three key time points: at diagnosis , after surgery, and at the time of relapse . The study included a Spanish cohort of 25 patients and an international validation cohort in Denmark with 15 patients, reinforcing the robustness and reproducibility of the results obtained.
Furthermore, the work integrated complementary techniques such as transcriptomic and proteomic analysis of tumor tissues , which allowed for a deeper understanding of the molecular mechanisms involved in tumor progression and immune system evasion.
One of the study’s key findings is that, in the early stages of colon cancer, the ability of tumor cells to evade the immune response could be a determining factor in patient relapse. This finding, described for the first time, opens a new avenue of research for the use of immunotherapy in early stages of colon cancer, a strategy that had not been considered until now.
Colon cancer is currently a huge challenge
Colorectal cancer represents one of the main challenges in oncology: it is the third most common tumor type worldwide and the second leading cause of cancer-related death, with nearly two million new cases each year. In Spain, more than 44,000 cases are diagnosed annually.
Currently, therapeutic decisions after colon cancer surgery are primarily based on pathological anatomy criteria. This approach has two major limitations: between 30% and 40% of patients considered cured end up suffering a relapse, and between 60% and 70% receive chemotherapy unnecessarily, with the resulting toxic effects and high healthcare costs.
To overcome these limitations, the research team developed a more precise, noninvasive, and widely applicable tool to identify early which patients are at real risk of relapse and allow for early intervention. This tool is the TAV16 test, which significantly improves the sensitivity of current techniques and has the added advantage of not requiring a sample of the primary tumor.
The TAV16 technology was compared with conventional methods, demonstrating significantly superior detection of residual disease, with a sensitivity ranging from 87% to 100%. Furthermore, it has high potential for technology transfer and is already patented.
__
(Featured image by National Cancer Institute via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in GACETA MEDICA. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us

-

 Fintech2 weeks ago
Fintech2 weeks agoSwissHacks 2026 to Launch Inaugural Swiss FinTech Week in Zurich
-

 Cannabis7 days ago
Cannabis7 days agoColombia Moves to Finalize Medicinal Cannabis Regulations by March
-

 Crowdfunding2 weeks ago
Crowdfunding2 weeks agoReal Estate Crowdfunding in Mexico: High Returns, Heavy Regulation, and Tax Inequality
-
 Markets2 days ago
Markets2 days agoMiddle East Escalation Sparks Market Uncertainty as Oil and Gold Poised to Rise