Biotech
Flomics Biotech Develops RNA-Based Blood Test for Early Cancer Detection
Flomics Biotech’s liquid biopsy detects five cancers—colon, lung, prostate, pancreas, and breast—with 92% accuracy. Using RNA biomarkers, AI, and next-generation sequencing, it identifies early-stage cancer with 80% sensitivity. Following promising study results, Flomics plans clinical trials and seeks funding to commercialize this minimally invasive, scalable early detection tool, revolutionizing preventive medicine.
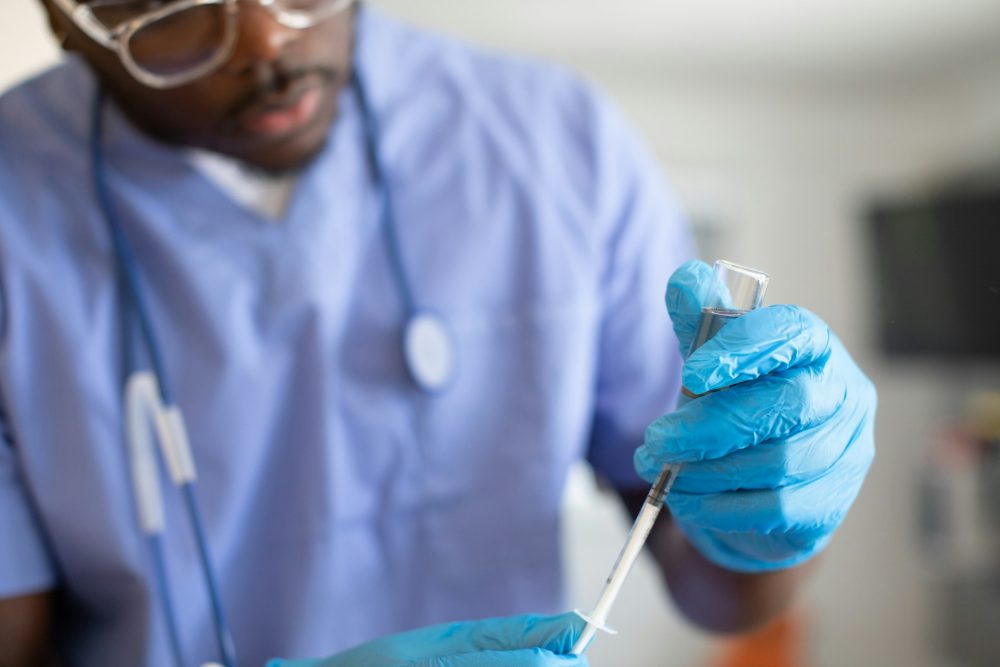
A liquid biopsy (blood test) developed by UPF Flomics Biotech is capable of accurately detecting five types of cancer, colon, lung, prostate, pancreas and breast in early stages.
That is the conclusion of a study presented by this emerging biotechnology company founded by former researchers from the Pompeu Fabra University (UPF) in Barcelona on this test based on a technology that uses cell-free RNA , next-generation sequencing (NGS) and bioinformatics driven by artificial intelligence.
The study, carried out with more than 1,000 blood samples, has a 92% probability of correctly differentiating between a sample with cancer and one without cancer, along with a sensitivity of 83% and a specificity of 90% .
Study in eight hospitals
Within the framework of the 10th Liquid Biopsy Symposium, held from 23 to 25 January in Santiago de Compostela, Flomics Biotech, a biotechnology start-up specialising in RNA-based liquid biopsy, has presented this important advance, which UPF reported on Friday in a press release.
The results of the latest study carried out with this blood test from different patients from eight hospitals , including the Hospital Clínic de Barcelona and the Andalusian Biobank, demonstrated the test’s ability to detect cancer in phase I, with a sensitivity of 80%, which reinforces its potential as a key tool for early detection, when treatments are most effective.
These results position Flomics at the forefront of RNA-based diagnostics and consolidate RNA as a state-of-the-art biomarker for multi-cancer screening; given that it is an accurate, minimally invasive and scalable test , and that in this case achieves 92% accuracy .
RNA as a biomarker
Flomics Biotech’s liquid biopsy is based on the RNA as a biomarker method. Conventional liquid biopsy methods (blood test) rely on circulating tumor DNA, which detects cancer mainly in advanced stages , when the tumor burden is more significant.
In contrast, RNA offers a real-time view of gene expression, capturing the dynamic molecular changes that occur in the early stages of cancer development. By integrating NGS and AI-driven bioinformatics, Flomics has developed a biomarker discovery platform , capable of identifying and analyzing RNA expression patterns associated with tumor presence, and providing superior sensitivity for early-stage cancers .
“These results confirm that RNA is a key biomarker for early cancer detection,” explains João Curado, CEO and co-founder of Flomics Biotech, who notes that “by leveraging advanced sequencing and artificial intelligence technologies, we have developed a highly accurate, minimally invasive and scalable liquid blood biopsy test , opening up new possibilities for early cancer detection on a large scale .”
Early detection improves survival
Flomics’ study focuses on five types of cancer (colorectal, lung, prostate, pancreatic and breast) and has shown promising results in all categories.
The ability to detect cancer in stage I, with a sensitivity of 80%, is particularly significant, as early detection dramatically improves survival rates. Flomics’ RNA technology offers a clear advantage over traditional methods, by capturing molecular alterations that DNA-based approaches do not detect , becoming a paradigm shift in preventive medicine.
Clinical trials, next step
Following the results of the study, Flomics will move forward to clinical trials, a crucial step towards regulatory approval and subsequent commercial launch.
As explained in the statement, large-scale validation studies are currently being prepared in collaboration with hospitals and research institutions to evaluate the performance of the test in real clinical settings. The company aims to bring its liquid biopsy technology to market, which will allow for the early detection of cancer through a simple blood test.
Flomics seeks funding
To support this next phase, Flomics is launching a new round of funding to accelerate clinical trials, regulatory approvals and commercial expansion. Having already raised over €4 million in private and public funding, Flomics is seeking additional investment to accelerate its entry into the healthcare sector.
“We are at a pivotal moment where our technology can redefine early cancer diagnosis,” adds Curado. “With the right partners and funding, we can bring this innovation to patients around the world, saving lives through early detection and better treatment options.”
__
(Featured image by Nappy via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in EL NACIONAL.CAT. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us

-
 Business1 week ago
Business1 week agoDow Jones Near Record Highs Amid Bullish Momentum and Bearish Long-Term Fears
-

 Business2 weeks ago
Business2 weeks agoDow Jones Breaks 50,000 as Bull Market Surges Amid Caution and Volatility
-

 Crowdfunding5 days ago
Crowdfunding5 days agoThe Youth Program at Enzian Shooting Club Is Expanding Thanks to Crowdfunding
-

 Impact Investing2 weeks ago
Impact Investing2 weeks agoEU Backs 90% Emissions Cut by 2040 and Delays ETS2 Rollout















