Biotech
Grünenthal closes a deal with Teva to market teriparatide
The number of old people suffering from osteoporosis is increasing every year. The companies Grünenthal and Teva partnered in order to market a product called Tetridar in the Spanish market, a medicine that would help people suffering from this problem. In Spain, 2.4 million people over the age of 50 suffered from osteoporosis in 2013. Grünenthal also launches the CuidarT Patient Support Program.

Grünenthal and Teva have reached an exclusive agreement for the marketing, distribution, and promotion of teriparatide (Tetridar) in the Spanish market. Both laboratories are once again joining forces to target patients suffering from osteoporosis. This agreement is expected to last until the end of 2027, with the possibility of being extended, according to information from the two companies.
João Simões, General Director of Grünenthal Pharma, wanted to put this type of partnership to work for the benefit of patients. “Thanks to this collaboration between the two companies, more people affected by osteoporosis and at high risk of fracture will see their quality of life improved. This disease and its consequences greatly affect those who suffer from it, since it causes pain and disability,” he said in a statement.
For his part, Carlos Teixeira, Teva’s general manager, expresses the company’s satisfaction: “It is important to weave alliances that help our drugs reach millions of people. In this case, hand in hand with a company like Grünenthal we want those people who suffer from osteoporosis to have good access to our product and can benefit from it.
If you want to read more about the agreement between Grünenthal and Teva to market Tetrida in the Spanish market and come in the help of people suffering from osteoporosis, download for free the Born2Invest mobile app. Available for both Android and iOS devices, our companion app brings you the latest business news in the world, so you don’t have to lose time searching the internet.
Teva and Grünenthal join forces again to address patients suffering from osteoporosis
This drug is a chemically synthesized drug, bioequivalent to the reference product (Forsteo), whose active ingredient is teriparatide. It is indicated in the treatment of osteoporosis in postmenopausal women and in men with an increased risk of fracture, as well as in osteoporosis associated with systemic therapy maintained with glucocorticoids. In Spain, 2.4 million people over the age of 50 suffered from osteoporosis in 2013.
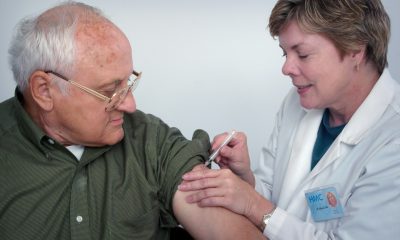
Teriparatide is a solution injected into a pre-filled pen and administered subcutaneously. It has been shown to significantly reduce the occurrence of vertebral and nonvertebral fractures in patients with osteoporosis. Likewise, the use of teriparatide can significantly relieve low back pain, improve the patient’s quality of life and increase mobility in patients with postmenopausal osteoporosis.
It is estimated that a new vertebral fracture occurs every 22 seconds worldwide, and the type of osteoporotic fracture is the most common. In addition, its incidence is increasing, mainly due to a longer life expectancy. That is why the two companies decided to partner to come in the help of people suffering from osteoporosis.
In Spain, 2.4 million people over the age of 50 suffered from osteoporosis in 2013
As a patient-centered pharmaceutical company, Grünenthal launches the CuidarT Patient Support Program. This program consists of training teriparatide patients in the proper administration of the drug, the use of the proper injection technique, and the correct use of the pen. In addition, Grünenthal provides these patients with an exclusive toll-free telephone number staffed by appropriately trained and experienced nurses.
__
(Featured image by Andrea Piacquadion via Pexels)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in iSanidad, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Crypto6 days ago
Crypto6 days agoCrypto Markets Under Pressure as Vitalik Buterin Sells 17,000 ETH
-

 Markets2 weeks ago
Markets2 weeks agoWeather-Driven Supply Outlook Lifts Coffee Markets in Brazil and Vietnam
-

 Impact Investing1 day ago
Impact Investing1 day agoGreen vs. Brown Stocks: Climate Policy, Capital Costs, and the Battle for Market Returns
-

 Business2 weeks ago
Business2 weeks agoTopRanked.io Weekly Affiliate Digest: What’s Hot in Affiliate Marketing [Best Technology Affiliate Programs]