Cannabis
Medical Cannabis Could Be the Future of Brain Cancer Treatment
The potential of cannabis to treat and even prevent certain types of cancer is an area of research that is growing rapidly. Cannabis-based products, both in anecdotes and in a growing number of scientific studies, are proving helpful to patients in a variety of ways, whether in managing palliative pain or reducing the side effects of standard therapies such as chemotherapy.

The start of 2023 brings new hope for patients with the UK’s most aggressive brain tumors. The first clinical trials have begun to test the effectiveness of Sativex, a cannabis-based drug, in treating glioblastoma multiforme.
The ARISTOCRAT study, being conducted at Leeds Teaching Hospitals NHS Trust and Christie NHS Foundation Trust in Manchester, is a pioneering global effort. It aims to test whether combining nabiximol (also known as Sativex) with chemotherapy can help prolong the lives of people suffering from recurrent glioblastoma multiforme.
Read more about the ARISTOCRAT study and find the latest cannabis news of the day with the Hemp.im mobile app.
Mobilizing researchers and the community
The study, led by researchers at the University of Leeds and the Cancer Research UK research unit at the University of Birmingham, plans to involve more than 230 patients with glioblastoma multiforme from 14 NHS hospitals in England, Scotland, and Wales.
All of this is possible thanks to a fundraising campaign run by The Brain Tumour Charity, which has managed to raise the needed £450,000 thanks to the support of Olympian Tom Daley.
Tough fight against glioblastoma
Glioblastoma multiforme is the most aggressive form of brain cancer, with an average survival time of less than 10 months after relapse. Currently, treatment options for people whose glioblastoma multiforme has returned are very limited, according to The Brain Tumor Charity.
A 2021 phase I study of 27 patients showed that nabiximol can be tolerated by patients in combination with chemotherapy, and more importantly, has the potential to extend the lives of people with recurrent glioblastoma multiforme. If the Phase II study confirms these results, experts hope that Sativex could become a new tool for patients with glioblastoma brain tumors – the first since the introduction of temozolomide chemotherapy in 2007.
What is Sativex
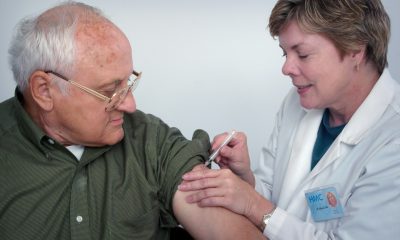
Sativex is a prescription drug designed to treat moderate to severe muscle spasticity in people struggling with multiple sclerosis. It is supplied as an oral spray, with a package containing 3 bottles of 10 ml each.
The Sativex formula contains two cannabinoids isolated from Cannabis sativa flowers – delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD). Their action focuses on the cannabinoid receptors CB1 and CB2, which are mainly located on nerve endings. This can affect neurotransmitter function, relieve muscle spasticity and improve mobility. After application of the Sativex spray, the active ingredients are quickly absorbed, and their traces in blood plasma are visible as early as 15 minutes after application to the oral mucosa. The cannabinoids then distribute throughout the body, accumulating in fatty tissue.
There are 27 mg/mL of THC and 25 mg/mL of CBD in 1 ml of Sativex. There are 2.7 mg of THC and 2.5 mg of CBD in every 100 microliters of aerosol. Additional ingredients include anhydrous ethanol, propylene glycol and peppermint oil.
Medical cannabis in the treatment of glioblastoma multiforme
Dr. David Jenkinson, scientific director of The Brain Tumor Charity, stresses the great importance of the research:
“We are very excited to launch this world-first study conducted in the UK, which has the potential to accelerate the fight against this devastating disease. Over the past decade, patients and researchers have shown great interest in the potential of cannabinoids to treat brain glioma. We are very grateful to everyone around the world who has contributed to funding such an important research effort.”
“It is encouraging to see the first results. We now look forward to answering the question of whether adding nabiximol to chemotherapy can improve the quality of life and prolong it for those affected by a diagnosis of glioblastoma multiforme. We are hopeful that this could be the first new drug to treat glioma in more than 15 years.”
Researchers want to see if adding Sativex to the current standard chemotherapy (temozolomide) can give patients with glioma recurrence after initial treatment additional time to live.
Study participants will be prescribed up to 12 doses of Sativex or placebo per day (or the maximum dose they can tolerate if lower than 12) administered orally. They will then be monitored on a regular basis, including by clinical assessment (every four weeks), blood tests, MRI scans (every eight weeks), and completion of quality-of-life questionnaires.
Is medical cannabis the answer to glioblastoma?
Professor Susan Short, principal investigator of the study at the University of Leeds, commented:
“We are very excited to launch this study here at Leeds. We look forward to conducting this study, which will show us whether cannabinoid-based drugs can help treat the most aggressive brain tumors. Treating gliomas is extremely difficult. Even after surgery, radiation, and chemotherapy, almost all of these brain tumors regrow within a year. Unfortunately, when this happens, the options for patients are very limited.”
Caution about cannabis-based therapies
The potential of medical cannabis to treat and even prevent certain types of cancer is an area of research that is growing rapidly. Cannabis-based products, both in anecdotes and in a growing number of scientific studies, are proving helpful to patients in a variety of ways, whether in managing palliative pain or reducing the side effects of standard therapies such as chemotherapy.
However, to date, there is no solid evidence of the effectiveness of using cannabis to treat brain tumors.
As Dr. Jenkinson pointed out: “For now, although some cannabinoid-based products may help alleviate symptoms, there is not enough evidence to recommend their use in the treatment of brain tumors. If you are considering using cannabinoid-based products or other complementary therapies, it is crucial that you discuss this with your medical team first, as they may affect other treatments, such as anti-epileptic drugs or steroids.”
Cannabis in the fight against the most aggressive type of brain cancer
Cannabinoid-based drugs have well-described effects on the brain, and their use in various types of cancer has long been of great interest. Glioblastoma multiforme has cannabinoid receptors on the surface of its cells. Laboratory studies on glioma cells have shown that these drugs can slow tumor growth and are particularly effective when used together with temozolomide.
“We now have the opportunity to use these laboratory results and the results from the Phase I clinical trial to see if this drug can help glioblastoma patients live longer as part of this randomized clinical trial, the first of its kind,” Professor Short emphasizes.
This study is particularly important because it could bring a breakthrough in the treatment of one of the most deadly forms of cancer. With current treatments, the survival rate of glioma patients after a relapse is less than 10 months. Sativex could prove to be a valuable tool in the fight against this disease, offering patients hope for a longer and better life.
__
(Featured image by CBD-Infos-com via Pixabay)
DISCLAIMER: This article was written by a third-party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in Fakty Konopne, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Crypto1 day ago
Crypto1 day agoMiddle East Tensions Shake Crypto as Bitcoin and Ethereum Slip
-

 Crypto2 weeks ago
Crypto2 weeks agoBitcoin Steady Near $68K as ETF Outflows and Institutional Moves Shape Crypto Markets
-

 Business1 week ago
Business1 week agoDow Jones Stalls Near Record Highs as Inflation-Fueled Rally Awaits Next Move
-
 Fintech5 days ago
Fintech5 days agoFirst Regulated Blockchain Stock Trade Launches in the United States