Biotech
Novo Nordisk: Ozempic Gets Positive Evaluation for Kidney Treatment
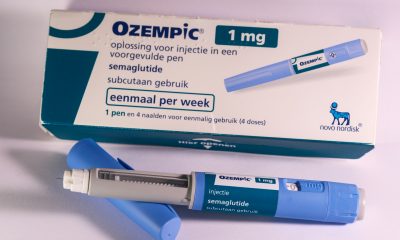
Ozempic, manufactured by Novo Nordisk, gains EMA approval for chronic kidney disease, reducing disease progression by 24%, per the Flow trial. With 40% of type 2 diabetes patients developing kidney issues, it’s the first GLP-1 receptor agonist for this use. Novo Nordisk’s Q3 revenue surged 48%, driven by Ozempic, Wegovy, and innovative therapies like CagriSema.

Ozempic continues to expand its footprint. Its manufacturer Novo Nordisk has announced that the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has approved the drug’s use for chronic kidney disease.
The Danish pharmaceutical company has presented the Flow trial, which evaluates the risk reduction of Ozempic therapy in events related to the disease. The trial demonstrated a statistically significant and superior reduction of 24% in the risk and progression of kidney disease .
The trial was launched because of the correlation between diabetes and these types of diseases. “Approximately 40% of people with type 2 diabetes develop chronic kidney disease , and there is a need for treatments that can help reduce the progression of kidney disease,” said Martin Holst Lange, executive vice president of development at Novo Nordisk.
Novo Nordisk boosts revenue by 48% in the third quarter, thanks to Ozempic and Wegovy
“With this positive opinion, Ozempic will become the first and only GLP-1 receptor agonist to show a reduction in the risk of kidney disease progression in adults with type 2 diabetes and chronic kidney disease,” the company added.
Novo Nordisk has also applied for an extension of the Ozempic label in the United States, with a decision expected in the first half of 2025. In parallel, the pharmaceutical company is working on a weight-loss pill.
The company is also developing another two-drug combination known as CagriSema, which also targets the hormone amylin. According to the pharmaceutical company, it has a weight loss potential of up to 25%.
Novo Nordisk closed the third quarter with revenue of $2.313 billion, up 48% from the same period last year. The company attributed the growth to sales of Wegovy, another weight-loss drug.
Novo Nordisk invests 1.14 billion euros in a new factory in Denmark
Novo Nordisk is strengthening its position in its home market. The Danish pharmaceutical giant has announced an investment of 8.5 billion crowns (1.14 billion euros) in a new factory in the city of Odense.
Specifically, the new plant will specialise in the production of medicines to treat rare diseases . Novo Nordisk acquired the plot last summer, although at the time it did not indicate what it would be used for.
The facility will house a warehouse and a factory. “The space will be designed to be modular and flexible and able to accommodate multiple types of products to treat rare diseases such as haemophilia,” Novo Nordisk said in a statement.
With its construction, the company is resuming the renewal of its production muscle in Denmark, after a century without opening new factories in the country. Novo Nordisk plans to have the plant operational in 2027 and will employ four hundred workers.
Novo Nordisk also announced another new factory in its home market this year
Last August, Novo Nordisk announced another investment in its home market, specialising in its pharmatech unit . The company will also strengthen its presence abroad with a €4 billion investment in a plant in the United States, dedicated to filling injection pens for Ozempic and Wegovy.
In parallel, the group received approval from the European Medicines Agency (EMA) last week to include properties for treating chronic kidney disease on the Ozempic label.
Novo Nordisk closed the third quarter with revenue of $2.313 billion, up 48% from the same period last year. The company attributed the growth to sales of Wegovy, another weight-loss drug.
__
(Featured image by Supliful via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in PlantaDoce and PlantaDoce. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us

-

 Africa6 days ago
Africa6 days agoMorocco’s Industrial Activity Stalls in January 2026
-

 Crypto2 weeks ago
Crypto2 weeks agoTariff Turmoil Sends Bitcoin and Ethereum Lower as Crypto Markets Face Mounting Pressure
-

 Crypto4 days ago
Crypto4 days agoBitcoin Surges Past $72K as Crypto Market Rallies and Kraken Secures US Banking License
-

 Crypto2 weeks ago
Crypto2 weeks agoEthereum Outlook: Key $2,190 Resistance, Whale Accumulation, and Buterin’s Push for True DeFi