Biotech
Abbott Presents Innovative Solutions to IDIS to Improve the Treatment of Mitral and Tricuspid Regurgitation
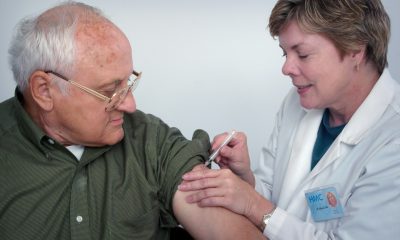
At an IDIS Foundation meeting, Abbott introduced two minimally invasive devices, MitraClip and TriClip, aimed at treating mitral and tricuspid regurgitation without open-heart surgery. Presented by Abbott and leading cardiology experts, these solutions improve patient recovery times and outcomes for complex cardiovascular diseases, enhancing patients’ quality of life and advancing treatment innovation.

Mitral and tricuspid regurgitation are cardiac pathologies that affect millions of people worldwide. In fact, they have a significant impact on the quality of life of patients and a high burden on health systems. It is estimated that mitral regurgitation affects approximately 4 million people while there are 1.6 million with tricuspid regurgitation. Abbott has developed tools to treat these two pathologies.
The prevalence of both pathologies, the associated complications and their undertreatment underline the need for tools for diagnosis and treatment to help improve the quality of life of patients. This was made clear at the meeting of the Innovation Committee of the Institute for the Development and Integration of Health ( IDIS Foundation ) where Abbott presented innovative solutions to improve the treatment of mitral and tricuspid regurgitation.
Abbott presented MitraClip and Triclip
Specifically, Mª José Blanco , director of the Structural Heart division of Abbott in Spain; Dabit Arzamendi , director of the Interventional Cardiology Unit at Hospital Sant Pau; and María Reyes, director of mitral and tricuspid business at Abbott, presented how new procedures are changing the future in the approach to both pathologies.
Two technologies were presented by Abbott during the meeting: MitraClip, which is responsible for repairing the mitral valve without the need for open heart surgery, and TriClip, an effective therapeutic alternative to conventional surgery for high-risk patients with tricuspid heart valve disease.
During the meeting, two of the technologies that Abbott works with were explained. On the one hand, the minimally invasive device MitraClip, which is responsible for repairing the mitral valve without the need for open heart surgery, reducing complications and the patient’s recovery time.
On the other hand, TriClip, which is offered as a therapeutic and effective alternative to conventional surgery for high-risk patients who suffer from this type of tricuspid heart valve disease. In addition, a presentation was made on the clinical results and the durability and safety of these technologies in the treated patients.
“The incorporation of new technologies in the field of cardiovascular health is essential to advance in the treatment of complex diseases such as mitral and tricuspid insufficiency. Devices such as those presented in this session allow us to offer more effective and less invasive solutions, which translates into a faster recovery and a significant improvement in the quality of life of patients in the long term,” said Ángel de Benito , director of operations of the IDIS Foundation.
__
(Featured image by Pexels via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in iSanidad. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us

-

 Crowdfunding2 weeks ago
Crowdfunding2 weeks agoThe Youth Program at Enzian Shooting Club Is Expanding Thanks to Crowdfunding
-

 Africa24 hours ago
Africa24 hours agoMorocco’s Industrial Activity Stalls in January 2026
-

 Crypto1 week ago
Crypto1 week agoTariff Turmoil Sends Bitcoin and Ethereum Lower as Crypto Markets Face Mounting Pressure
-

 Crypto6 days ago
Crypto6 days agoEthereum Outlook: Key $2,190 Resistance, Whale Accumulation, and Buterin’s Push for True DeFi