Biotech
Abbott’s MitraClip helped patients with severe heart failure
Abbott presented positive results for its MitraClip device which could help patients with severe heart failure.

Researchers reported on Sunday that a tiny clip inserted into the heart was able to reduce the mortality rates in patients with severe heart failure, per The New York Times. The study was published in the New England Journal of Medicine and was also presented at the 30th Transcatheter Cardiovascular Therapeutics (TCT) annual scientific symposium of the Cardiovascular Research Foundation in San Diego.
The device, which is called MitraClip, will be used to clip the two flaps of the mitral valve, eventually repairing it. MitraClip was manufactured by Abbott. The company also funded the study, while independent experts reviewed the trial data.
Medical experts found that the patients in the clinical trial were able to avoid additional hospitalizations. Their quality of life also improved because of lesser symptoms.
Until after the results, the researchers did not know that fixing the mitral valve would improve the health of the patients.
The study included 614 patients with severe heart failure from Canada and the United States. They were randomly assigned to receive MitraClip together with the standard treatment or to proceed with the usual treatment alone.
Among those who received the device, only 92 were hospitalized, while of those who only received the standard treatment, 151 were hospitalized. During the same period, 28 patients on the group that received the device died, while 61 patients from the group that received the usual treatment died.
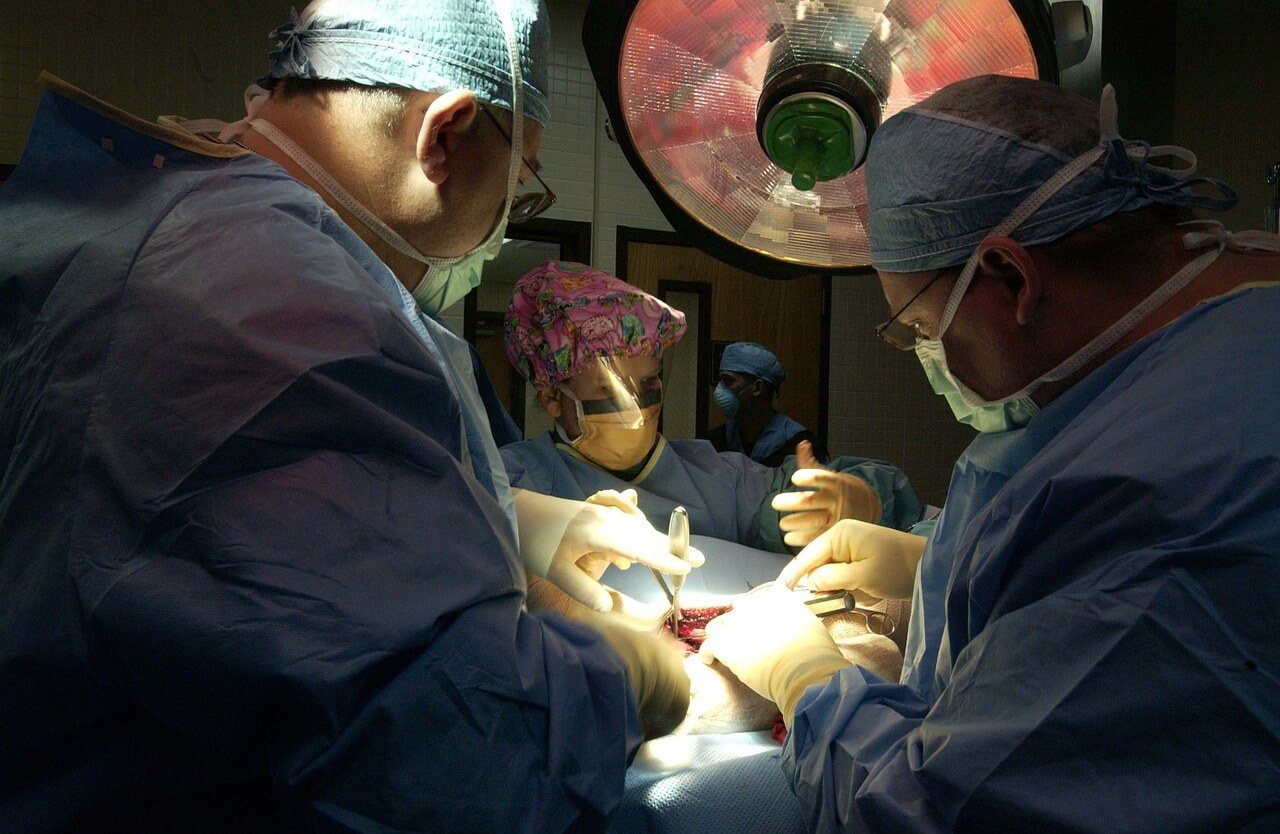
Designed to lower the mortality rates in patients with severe heart failure, the MitraClip needs to be threaded through a blood vessel and into the heart. (Source)
The attachment of the clip is quite tricky. A cardiologist needs to thread the device to the heart through a blood vessel in the groin—through a minimally invasive catheter. Once the clip reaches the heart, it should be guided to the valve. Then, the device will be used to clip the two flaps of the valve together.
Because of the difficulty of the procedure, not all cardiologist can do the process. They need the training to develop expertise in this area and dedication in order to do the job.
The device has already been approved by the U.S. Food and Drug Authority (FDA) for patients who are too fragile to go under the knife. If approved for all patients with severe heart failure, insurers are likely to cover it, per The Business Times. The device has already been used in Europe, but there are no studies yet about the device that really helped its cause.
According to the Center for Disease Control and Prevention, there are around 5.7 million adults with heart failure in the U.S. About half of them die within five years of diagnosis. The usual symptoms of heart failure are weakness, fatigue, irregular or fast heartbeat, coughing and wheezing. There are several types of heart failure. MitraClip could help patients with mitral regurgitation—leakage of blood through the mitral valve every time the left ventricle contracts.
The MitraClip received CE Mark in Europe in 2008, and as mentioned, it was also approved by the FDA in 2013 for high-risk patients, per MarketWatch. This game changer device currently costs $30,000, excluding hospital costs and professional fees.

-

 Crypto6 days ago
Crypto6 days agoEthereum Outlook: Key $2,190 Resistance, Whale Accumulation, and Buterin’s Push for True DeFi
-
 Cannabis2 weeks ago
Cannabis2 weeks agoAI Can Mimic Psychedelic Experiences but Cannot Truly Feel Them, Study Warns
-
 Biotech20 hours ago
Biotech20 hours agoShingles Vaccine Linked to Significant Reduction in Dementia Risk
-

 Crowdfunding1 week ago
Crowdfunding1 week agoBSG Stahl Riesa Launches Crowdfunding for New Floodlights























You must be logged in to post a comment Login