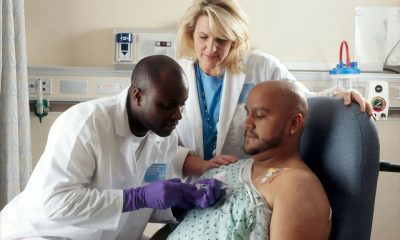
Biotech
The German biotech sector lags behind in the development of novel gene therapies
Germany is not yet particularly well positioned in research and development of novel gene and cell therapies. Most studies are carried out in the USA (48%) and China (39%). Although Germany ranks third, this does not make an impression given that only around four percent of all clinical ATMP projects are carried out in Germany. Vfa bio makes a few recommendations for the development of the sector.

Is Germany keeping pace with the latest biotechnological development trends? According to the Association of Research-Based Pharmaceutical Companies, or vfa bio, there is a need for action.
Within a decade, the share of recombinant drugs in the German pharmacy and hospital market has almost doubled and the manufacturer’s turnover has almost tripled. In 2019, the market share of red biotechnology was 29 percent (2009: 16 percent) with total sales of $14.3 billion (€12.7 billion) (+13 percent, at ex-factory prices). That was reported by Dr. Frank Mathias, Chairman of the vfa bio interest group, in Frankfurt on Tuesday, June 30th.
Read more about the development of the biotech sector in Germany and be the first to find out the latest business news in the world with our companion app. Download the Born2Invest mobile app for free and read about the latest discoveries in the biotech sector.
Germany should involve more in the research and development of novel gene therapies
According to the report, biosimilars, which exceeded the billion euro mark for the first time, grew disproportionately strongly in the year under review – and with $1.68 billion (€1.5 billion) in manufacturer sales they did so significantly (+60%).
The biotech industry is also attracting particular attention due to the recent approval successes of novel gene and cell therapies, which are summarized in industry jargon as “Advanced Therapy Medicinal Products” under the abbreviation ATMP. Ten such therapies have been approved in the EU since 2015, and many more are expected to follow. According to the Boston Consulting Group (BCG), more than 1,000 phase I to III study projects worldwide are working on new gene or cell therapies.
“Do we want to participate or leave it to others?” said Dr. Frank Mathias, Chairman of vfa bio. A rhetorical question on the promotion and testing of new gene and cell therapies in Germany.
Mathias cited the coagulation factor IX replacement therapy Fidanacogen Elaparvovec, which is currently being tested by Pfizer and Spark Therapeutics in phase III, as an example of one of the next innovations in this field. Spark, as the developer of the gene therapy Luxturna (Voretigen Neparvovec, together with Novartis), is one of the pioneers of the ATMP.
According to Mathias, Germany is not yet particularly well positioned in research and development of novel gene and cell therapies. Most studies are carried out in the USA (48%) and China (39%).
Although Germany ranks third, this does not make an impression given that only around four percent of all clinical ATMP projects are carried out in Germany. Among the recommendations that vfa bio makes to Berlin in order to trim the location to an, so literally, “ATMP welcome culture” are: to establish a research center for gene and cell therapies modeled on the DKFZ, to establish an “ATMP Task Force” to harmonize the requirements for clinical trial projects across countries, and to provide the Paul Ehrlich Institute with more staff in order to shorten the approval period for clinical studies.
In addition, the use of these innovative therapies must be reimbursed to the clinics more quickly. The current option of agreeing on additional fees for “new examination and treatment methods” (NUB) is too cumbersome and restrictive.
__
(Featured image by qimono via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in ArzteZeitung, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Cannabis3 days ago
Cannabis3 days agoCannabis Company Adopts Dogecoin for Treasury Innovation
-

 Biotech1 week ago
Biotech1 week agoPfizer Spain Highlights Innovation and Impact in 2024 Report Amid Key Anniversaries
-

 Markets5 days ago
Markets5 days agoStock Markets Surge Amid Global Uncertainty, But Storm Clouds Loom
-
 Africa2 days ago
Africa2 days agoMorocco Charts a Citizen-Centered Path for Ethical and Inclusive AI