Biotech
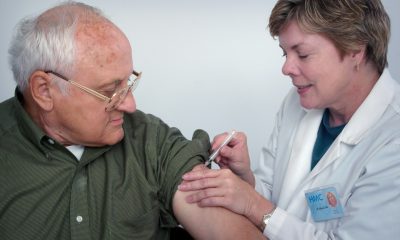
Biophytis is working on a treatment for respiratory failure linked to COVID19
The Sarconeos clinical program should soon be tested at the Pitié-Salpêtrière Hospital in patients with coronavirus. This active molecule of natural origin is already being tested in sarcopenia, a form of age-related muscle degeneration. Another goal of the experimental treatment is to halt respiratory deterioration in patients with Duchenne muscular dystrophy.
Direct intervention with hospitalized patients suffering from COVID-19 to prevent respiratory failure – which requires the use of the famous ventilator, or even admission to intensive care units – is the objective of the new clinical program set up by Biophytis. The French biotechnology company is specializing in the development of treatments for neuromuscular diseases, in collaboration with the Pitié-Salpêtrière hospital in Paris.
Founded in 2006 and listed on Euronext Paris in 2015, the company announced Tuesday morning, April 7th, the launch of a new clinical development program called COVA. The program is based on the company’s flagship compound Sarconeos (BIO101) as a potential treatment for respiratory failure associated with COVID-19.
This program, through a phase 2/3 trial (a clinical trial that could lead directly to the submission of an application for approval to the drug agency prior to marketing) will consist of evaluating the therapeutic efficacy and safety of Sarconeos (BIO101) in the treatment of acute respiratory distress syndrome (ARDS), the most lethal symptom associated with COVID-19. As soon as the National Agency for the Safety of Medicines (ANSM) gives the go-ahead, the trial can begin, starting with Pitié-Salpêtrière, before being extended to Belgium and the United States in particular.
Sarconeos is an experimental treatment
Sarconeos is an experimental drug derived from medicinal plants called phytoecdysteroids, used as toning agents in various pharmacopeias. This compound was first tested for its ability to improve muscle function in frail elderly patients with sarcopenia, a form of age-related muscle degeneration. The disease is affecting, depending on the source, between 6% and 22% of people over 65 years of age. In addition, Biophytis recently received the green light for a phase 2/3 study to test its efficacy in preserving the respiratory function of children with Duchenne muscular dystrophy.
“COVID-19 has completely transformed our society on a global scale and has had a devastating effect, particularly on the health of the frail elderly. We wish to respond to the call from our industry and national and international organizations to join the global effort to fight this pandemic. Sarconeos (BIO101) has demonstrated its ability to restore normal lung function in several experimental models by activating the renin-angiotensin system, the very system that is attacked by the SARS-Cov-2 virus. In the absence of a vaccine and antiviral treatment with proven efficacy, it could offer a drug treatment for acute respiratory failure in hospitalized COVID-19 patients. Sarconeos could potentially limit the use of mechanical ventilation and promoting people’s potential recovery,” said Stanislas Veillet, co-founder and CEO of Biophytis.
“We hope for a favorable response from the ANSM to be able to start this study in France in order to respond as quickly as possible to this health emergency,” Veillet added.
At the same time, the company announced a strengthening of its financial structure through the refinancing of its current bond issue, on more advantageous terms, and an issue of share subscription warrants (BSA), with a priority period granted to the company’s current shareholders. The latter will be able to subscribe to 3 BSAs for 16 shares held on April 8th, each BSA then allowing them to subscribe over the next five years to one new share at a unit price of €0.27 for a period of 5 years from the date of settlement and delivery, at an exercise price of €0.27 (17% above the last closing price).
__
(Featured image by fernandozhiminaicela via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in TradingSat, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-
 Fintech5 days ago
Fintech5 days agoFirst Regulated Blockchain Stock Trade Launches in the United States
-

 Africa2 weeks ago
Africa2 weeks agoAir Algérie Expands African Partnerships
-

 Cannabis1 day ago
Cannabis1 day agoAurora’s Electric Honeydew Debuts in Poland, But Shared Registry Raises Patient Caution
-

 Markets1 week ago
Markets1 week agoRising U.S. Debt and Growing Financial Risks