Biotech
Galicia Becomes First in Spain to Approve Gene Therapy for Hemophilia B
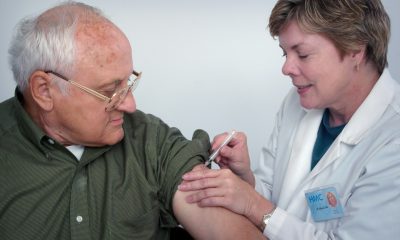
Galicia has approved a €15.6 million investment to supply gene therapy for hemophilia B, becoming the first region in Spain to do so. The therapy, Etranacogene Dezaparvovec, offers a potential cure for the rare genetic disorder. Eight of 51 diagnosed patients in Galicia are expected to qualify under strict national clinical and pharmaceutical criteria.

The President of the Xunta, Alfonso Rueda, announced on Monday that the regional government of Galicia has approved an investment of €15.6 million for the purchase of the first gene therapy drugs for treating hemophilia B.
He emphasized that the tender for the supply of the drug Etranacogene Dezaparvovec, a gene therapy treatment for the hereditary blood coagulation disorder, affirms “the validity of public health” and positions Galicia as “the first community in Spain to treat hemophilia B with gene therapy.”
Galicia Invests €15.6 Million to Launch Groundbreaking Gene Therapy Treatment for Hemophilia B Patients
Rueda highlighted that prior to the development of this therapy, hemophilia B had no curative treatment. The initial contract for the drug’s supply will last 24 months, with the possibility of extending it for an additional two years.
Hemophilia B (HB) is a rare, genetically inherited disease with an incidence rate of five cases per 100,000 males. It is linked to the X chromosome and caused by a deficiency or absence of factor IX. Although it primarily affects men, women carrying the mutation may also show symptoms of the condition.
The General Directorate of the Basic Portfolio of Services of the National Health System and Pharmacy has approved the inclusion of this gene therapy in the national pharmaceutical provision. However, its application is limited to treating adults with severe or moderately severe hemophilia B. These patients must have no history of inhibitors to factor IX, must have received stable prophylactic treatment with extended-life recombinant factor IX for at least two years, and must present anti-capsid neutralizing antibody levels against AAV5 equal to or below 1:678.
Rueda noted that there are currently 51 individuals diagnosed with hemophilia B in Galicia—37 men and 14 women. Among these, it is expected that eight patients may be eligible to receive gene therapy, provided they meet all other clinical inclusion criteria specified in the Etranacogene Dezaparvovec Pharmacoclinical Protocol.
Galicia’s investment and treatment initiative mark a significant milestone in the application of gene therapy within Spain’s public healthcare system and represents a step forward in improving care for patients with rare genetic disorders.
__
(Featured image by Sangharsh Lohakare via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in diariofarma. A third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us

-

 Impact Investing2 weeks ago
Impact Investing2 weeks agoItaly’s Listed Companies Reach Strong ESG Compliance, Led by Banks and Utilities
-

 Fintech4 days ago
Fintech4 days agoFindependent: Growing a FinTech Through Simplicity, Frugality, and Steady Steps
-

 Impact Investing2 weeks ago
Impact Investing2 weeks agoCDP Approves €1.5 Billion Package to Boost Industry, Renewables, and International Development
-

 Impact Investing6 days ago
Impact Investing6 days agoThe Sustainability Revolution: Driving a Net-Zero, Nature-Positive Economy