Biotech
Biotechs: Is France giving itself the means to achieve its ambitions?
The Strategic Council for Health Industries is making plans to turn France into the number one destination for emerging biotech companies. The council will reduce the risks of investing in new biotech companies by issuing world-class grants. This tactic will trigger a biotech revolution in France and help the country remain an important player in the pharmaceuticals sector.
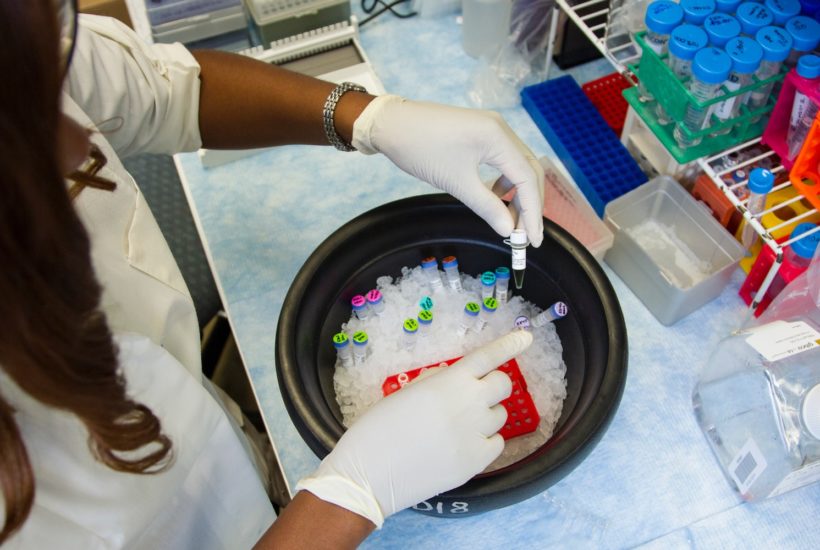
It is not normal for biotech start-ups to be bought out by “big pharma.” “We must be able to give them the means for their own organic growth,” wrote Gérard de Pouvourville. If the government wants to establish a thriving French biotech industry they need to be willing to pay the price to support it.
In its 2018 report, the Strategic Council for Health Industries has high ambitions: to make France a home for emerging biotech companies, both French and foreign. At the same time, however, the Social Security Financing Plan 2020 programs a $1.4 billion (€1.3 billion) saving on the price of medicines and medical devices.
Access to the reimbursed market in France is guided above all by the concern to make available to patients products with a proven risk-benefit ratio. These medicines need to be based on rigorous evidence, at a price that is sustainable for the national Health Insurance programmes. But is this rigor compatible with real industry policy, at a time when the pharmaceutical sector is undergoing major changes and is experiencing a third biotech revolution?
The Born2Invest mobile app is providing the latest bioscience industry news, business headlines of biotech news related to biotech research laboratories, biotech companies, biotech institutes.
Biotechnology transformed the pharmaceutical sector
Biotechnology, which has transformed the pharmaceutical sector, is at the dawn of a new revolution. Today, breakthrough technologies such as cell-based therapies and different approaches to gene therapy are arriving. Many of these innovations are being developed by a new generation of companies. Established players in the pharmaceutical industry rely on these biotechs to innovate. In its 2018 report, the OECD observed that over the period 2010-2012, big pharma companies held 63% of marketing authorizations but that their in-house research contributed only 30% of the total number of marketing authorizations.
Why would innovative companies decide to sell their technology – or even eventually their company – rather than become the new Sanofi? By choice perhaps, by necessity more certainly. Indeed, organizations deciding on access to reimbursed markets are faced with highly innovative technologies, high unit prices, and significant clinical and economic uncertainties.
An increased commercial risk
In this context, emerging biotechs face an increased commercial risk, that of delayed access or even no access at all. The implications of failure are drastic, as these companies often have no source of revenue. Their sustainability and that of their research pipeline depend on their ability to launch their first products in order to generate the revenues needed to repay their debts but also to invest in further innovation. Although they are at the origin of most of the recent pharmaceutical revolutions, many innovative biotechs are therefore often forced to relinquish their licenses or sign co-development partnerships with big pharma.
The takeover of small businesses by established companies can be considered normal in a competitive world. But it is also a cause for concern. Indeed, the acquisition of companies can lead to a risk of outbidding and excessively high acquisition costs, which can result in high drug prices.
Is it, therefore, inevitable that young biotechs will only be absorbed by a large group? Or can they also hope to grow organically? For a country like France, this is a considerable industrial challenge recognized by the public authorities, in terms of scientific, technological and clinical expertise, but also in terms of job creation and exports. We are entitled to wonder whether it is not strategically and politically more interesting to support investment, competition and the creation of jobs and companies, rather than the outsourcing of risk and the internalization of profits by established companies.
For the government, it is time to finally make a break with twenty years of (lack of) branch policy for the biotech sector. This means inventing a new doctrine of market access and pricing.
__
(Featured image by National Cancer Institute via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in LesEchos, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Crypto1 week ago
Crypto1 week agoMiddle East Tensions Shake Crypto as Bitcoin and Ethereum Slip
-

 Business2 weeks ago
Business2 weeks agoDow Jones Stalls Near Record Highs as Inflation-Fueled Rally Awaits Next Move
-

 Cannabis4 days ago
Cannabis4 days agoCanopy Growth Launches Cheaper 15g Medical Cannabis in Poland
-
 Fintech2 weeks ago
Fintech2 weeks agoFirst Regulated Blockchain Stock Trade Launches in the United States