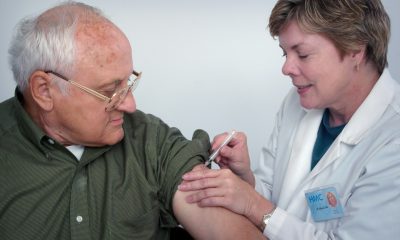
Cannabis
Why cannabis regulations might put a strain on the development of the industry in Mexico
The regulations for the medical use of cannabis contain a series of guidelines that restrict research and limit access to products to patients who require them. Industry players and activists estimated that Mexico has the potential to develop a market worth $5 billion and create up to 75,000 jobs with the approval of the cannabis law that is currently being discussed in the Senate.

The medical cannabis industry may see some storms before it flourishes. Although it was expected that with the regulation on the medicinal use of cannabis Mexico would take the first steps to become a power, there are some limitations for the sector.
The rules for pharmacological research, the issuing of specific prescriptions for these products, and the registration of patients are some of the points that could create bottlenecks for the sector, agree experts consulted by Expansión.
The Ministry of Health and the Federal Commission for Protection against Health Risks (Cofepris) published these regulations on January 12th, three years after the use of medical cannabis became legal, and they deal with aspects related to the regulation, control, promotion, and health surveillance of cannabis as raw material, its pharmacological derivatives, and medicines.
Although the regulation published by the authorities in the Official Journal of the Federation (DOF) gives the green light to medical research on cannabis products, this will not allow more to be available on the market in the short term because they have to be developed under the protocols created for pharmaceutical companies, i.e., they must have extensive clinical research that takes years to develop.
Raul Elizalde, global CEO of HempMeds, explained that under these guidelines products made from cannabis must become medicines, a process that has been so complex that there is only one authorized by the U.S. authorities on the market: Epidiolex, made from CBD, a cannabidiol found in hemp, which controls seizures related to two rare and severe types of epilepsy.
Find more about the legalization of cannabis in Mexico and read the most important cannabis news with the Hemp.im mobile app.
The law to regulate cannabis limits the development of the hemp industry
“The problem is that it is very complex in the pharmaceutical industry, especially in relation to cannabis. Today you can already do scientific research, but it is very difficult to register a botanical product as a medicine, because the molecule (of the medicine formula) is not synthetic,” he stated.
Elizalde (the father of Grace, the girl with epilepsy who opened the way for the medicinal use of cannabis) said that in other countries of the world, such as the United States, the regulation of the medicinal use of cannabis is adapted to the herbal and therapeutic uses of cannabis rather than a strictly pharmaceutical use, so “Mexico lost that opportunity in the pharmaceutical regulation.”
The businessman from Monterrey believes that the authorities should have considered the model that operates in Brazil, which allows the development of the product and have a pre-registration to then develop clinical studies and perform the registration, according to the result.
The size of the medical cannabis market in Mexico will reach a value of $50 million by 2024 from an estimated value of $3.5 million this year, according to the German consulting firm Statista.
The move to the gray and black market
Lorena Beltrán, CEO of CannabiSalud, a firm specialized in organizing forums and events to promote and inform about cannabis, added that by not considering the herbal characteristics of products with less than 1% THC, the psychotropic active ingredient of cannabis, such as food supplements, herbal remedies or food or beverages, will no longer have the characteristic of free sale that they obtained when the Congress of the Union approved the medicinal use of cannabis, in 2017. “Unfortunately it is a regulation that comes too restrictive, even than the law itself,” he said.
Added to this is that in order to prescribe a medicine with CBD, doctors, dentists or veterinarians must have a prescription book authorized by Cofepris, which will have a barcode, while consumers must be registered in pharmacies authorized to market the drugs.
“This is super unfortunate, because it does not give quick access to medicinal products that patients need (…) We thought it was going to solve this problem and no, we fall again in the same, in restrictions where the patient and his family have to keep going to a market or gray or a black market,” said Beltrán.
The legalization of cannabis could turn Mexico into a production powerhouse
The interviewees agreed that, although the regulation for the medicinal use of cannabis has already been established, legislators should look at the legislation of other countries to learn what has worked and, above all, make a distinction between the medicinal, recreational and industrial industries. However, more than the pharmaceutical industry, the detonator of the economy will be hemp, -the plant’s fiber-, from which textiles, ecological fuels, bio-construction materials, cellulose for paper and cosmetics are made.
“Currently, hemp is the product that Mexico can be ready to start working in the entire industry production chain, from planting, maquila products, domestic sales and export to other countries such as the United States and Europe, which already have a very regulation where other products such as food, cosmetics, supplements and textiles, fibers are already being sold,” said Raul Elizalde.
Industry players and activists estimated that Mexico has the potential to develop a market worth $5 billion and create up to 75,000 jobs with the approval of the cannabis law that is currently being discussed in the Senate and could be approved by the end of April.
__
(Featured image by jorono via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in EXPANSION, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Crypto1 day ago
Crypto1 day agoMiddle East Tensions Shake Crypto as Bitcoin and Ethereum Slip
-

 Crypto2 weeks ago
Crypto2 weeks agoBitcoin Steady Near $68K as ETF Outflows and Institutional Moves Shape Crypto Markets
-

 Business1 week ago
Business1 week agoDow Jones Stalls Near Record Highs as Inflation-Fueled Rally Awaits Next Move
-
 Fintech5 days ago
Fintech5 days agoFirst Regulated Blockchain Stock Trade Launches in the United States