Biotech
French Biotech SparingVision Raises a Large Sum of Money
Initial trial results for SPVN06 are expected in 2023, with first efficacy data expected in 2025. SparingVision is also developing SPVN20, a second gene therapy drug candidate that is independent of gene mutations. This one aims to restore vision in patients with advanced forms of retinitis pigmentosa and is expected to enter clinical trials in 2024.
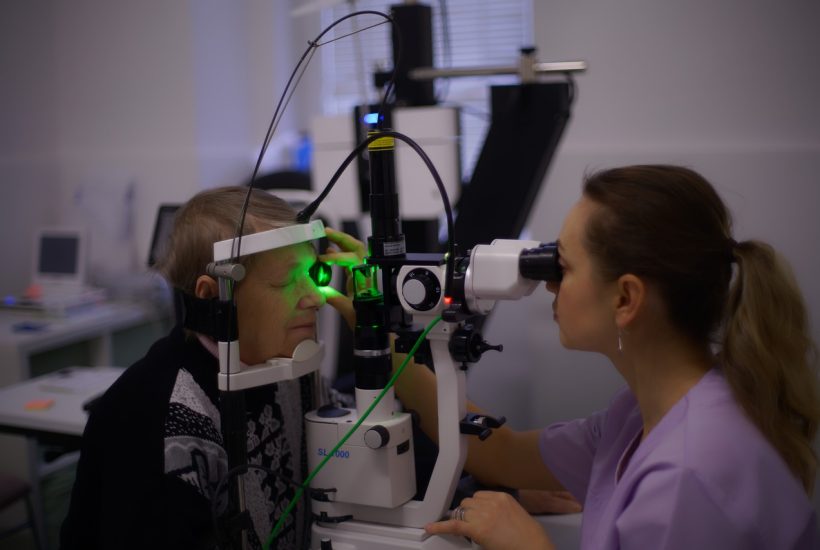
French biotech company SparingVision, which specializes in innovative therapies for retinal diseases, has raised €75 million to develop its two main products, it said Wednesday, September 14th. Founded in June 2016, SparingVision, an offshoot of the Vision Institute in Paris, will use the funds to finance the first clinical studies of its two main gene therapy treatments, one of which is scheduled to start a trial by the end of the year.
The latter, SPVN06, its most advanced potential treatment, is aimed at combating retinitis pigmentosa, a degenerative genetic disorder of the eye. This inherited disease, which can be caused by mutations in more than 70 genes, is characterized by a progressive and gradual loss of vision leading to blindness. It affects two million patients worldwide, including some 40,000 in France.
Born2Invest mobile application is bringing all the biotech and business news from trusted sources to a single screen so you can stay on top of the market. The application is aggregating the most important and breaking news from relevant websites, the list is always revised and updated with new resources.
SparingVision’s initial trial results for SPVN06 are expected in 2023
With its treatment, SparingVision seeks to provide retinal photoreceptors with the proteins they need to survive, by having them manufactured by cells other than the patient’s own deficient cells, the company’s CEO Stéphane Boissel told AFP. “This treatment should work regardless of the genetic mutation that caused the disease in the first place, unlike treatments proposed by competitors,” Boissel said. Only one treatment is currently marketed for this pathology, Luxturna, acquired by the Swiss group Roche and intended for patients with a specific genetic mutation.
Initial trial results for SPVN06 are expected in 2023, with first efficacy data expected in 2025. SparingVision is also developing SPVN20, a second gene therapy drug candidate that is independent of gene mutations. This one aims to restore vision in patients with advanced forms of retinitis pigmentosa and is expected to enter clinical trials in 2024.
In addition, the funds raised will accelerate the development of SPVN50, a genome-editing drug candidate developed in partnership with Intellia Therapeutics, a US biotech company specializing in CRISPR/Cas9 molecular scissors. SparingVision has secured funding from French and American funds Jeito Capital and UPMC Enterprises, 4BIO, Bpifrance, RD Fund, the Fighting Blindness Foundation’s venture capital fund, and Ysios Capital. The biotech company had already raised €60 million in a previous transaction.
__
(Featured image by newarta via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in Capital, a third-party contributor translated and adapted the articles from the originals. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Crypto1 week ago
Crypto1 week agoThe Crypto Market Rally Signals Possible Breakout Amid Political Support and Cautious Retail Sentiment
-

 Crypto4 days ago
Crypto4 days agoBitcoin Hits New Highs in USD, But Euro Investors See Limited Gains
-

 Crypto2 weeks ago
Crypto2 weeks agoXRP vs. Litecoin: The Race for the Next Crypto ETF Heats Up
-
 Crypto1 day ago
Crypto1 day agoCrypto Markets Surge on Inflation Optimism and Rate Cut Hopes

























