Biotech
Is a breakthrough lung cancer treatment on the horizon?
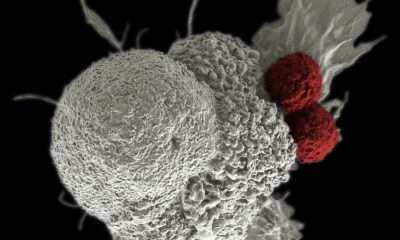
The Liège-based biotech company PDC*line Pharma, which specializes in immuno-oncology, has initiated a Phase I/II study to evaluate a therapeutic vaccine against lung cancer. This cancer variety causes the highest number of deaths in the world. The company has raised $18.9 million (€17 million) in equity and loans from Belgian investors and several angel investors.
A new Belgian biotech company is moving its projects out of the laboratory and into the clinical study phase. The product in question, called at this stage PDC*lung 01, is a therapeutic candidate vaccine against so-called “non-small cell” lung cancer (NSCLC), the most common lung cancer (80% of cases). Unlike traditional vaccines, which prevent disease, therapeutic cancer vaccines are designed to treat existing cancer and reduce potential growth.
Nine clinical centers, including three in Belgium, have agreed to participate in this clinical study. “This Phase I/II should be used to check the safety of the product, but should also look at whether there is an immune response and whether the vaccine is active in the blood. We will also observe if there are any clinical applications for the product,” continued the company’s CEO.
Born2Invest is a digital media website covering the biotech industry, among others. Our companion app allows you to get your daily dose of financial news from industries like banking, cannabis, innovation in biotechnology, and many others.
Lung cancer is the most common cancer among men and for both sexes combined, with 2.1 million new cases each year. For women, it is the third most common cancer, after breast and colorectal cancer. Lung cancer is the leading cause of cancer deaths worldwide, with 1.8 million people dying in 2018.
This represents 18.4% of all cancer deaths, both male and female. “We started with the lung because it is one of the cancers that require the most urgent treatment tools. Studies go faster because it is easier to find patients to enroll than for other rare varieties of cancers,” Eric Halioua noted.
Originally created in France
Eric Halioua is not a stranger to Belgian biotechs since he is the former boss of Promethera, another major name in Walloon cell therapy, specializing in the treatment of liver diseases. It was he who brought PDC*line to Liege in 2016. The company was initially founded in France in 2014 by Laurent Levy and Joël Plumas, as a spin-off of the French Blood Establishment in Grenoble.
Located in the Giga building in Sart Tilman, PDC*line has about twenty employees. The young company has raised $18.9 million (€17 million) to date, including $8.46 million (€7.6 million) in equity and loans from Belgian investors (MeusInvest, Innodem3, InvestSud, and SFPI) and several business angels, including Jean-Paul Prieels (ex-GSK).
The rest consists of $10.36 million (€9.3 million) of non-dilutive investments, including recoverable advances and subsidies from the Walloon Region and French and Belgian organizations.
Without giving any details, Eric Halioua indicated that he was in the process of raising new funds, which could materialize quite quickly. At the beginning of the year, the company granted LG Chem Life Sciences Company of Korea an exclusive license to develop and market PDC*lung01 for South Korea and an exclusive option in other Asian countries. A contract worth $120 million (€108 million), plus royalties. An impressive amount for such a young company.
__
(Featured image by Dmitry Bayer via Unsplash)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in L’echo, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Cannabis1 week ago
Cannabis1 week agoWhen a Cutting Becomes a Cannabis Plant: Court Clarifies Germany’s Three-Plant Rule
-

 Africa2 weeks ago
Africa2 weeks agoIvory Coast Development Plan 2026–2030: Investment, Growth, and Strategic Reforms
-
 Africa3 days ago
Africa3 days agoMASI Surge Exposes Market Blind Spot: The SAMIR Freeze and Hidden Risks
-

 Crypto1 week ago
Crypto1 week agoBitcoin Rebounds Above $70K as Crypto Markets Show Fragile Signs of Recovery