Biotech
LentiStem seeks more than half a million euros to advance gene therapy
LentiStem seeks more than half a million euros to advance gene therapy. The biotech company has raised $311,000 (€260,000) in public capital and is seeking to raise more than $480,000 (€400,000) to complete the preclinical phase in the third quarter. LentiStem aims to improve the design and development of unique tools to modulate the activity of Car-T cells focused on cancer treatment through immunotherapy

LentiStem is seeking capital. The Spanish company wants to raise more than half a million euros in 2021 to advance gene therapy. The technology-based company focuses on the research and development of innovative therapies based on gene regulation and gene transfer systems, with special emphasis on cancer immunotherapy.
The biotech has recently attracted $311,000 (€260,000) from an industrial doctorate from the Ministry of Science and Innovation and the Neotec program of the Center for the Development of Industrial Technology (Cdti). The company is seeking another $167,000 (€140,000) of private capital to complete the project selected by Neotec, according to Carlos Martín, CEO of Lentiste.
In addition, the company wants to access more funding after this project to move forward with preclinical and clinical trials. LentiStem aims to raise more than $480,000 (€400,000) to complete the preclinical phase of its studies in the third quarter of 2021. In total, the biotech requires more than half a million euros to advance its technology.
Read more about LentiStem and its plans to advance gene therapy and find the latest business headlines with our companion app, Born2Invest.
LentiStem seeks $167,000 (€140,000) to finish the project selected by Neotec
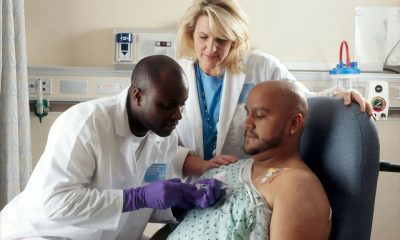
LentiStem aims to improve the design and development of unique tools to modulate the activity of Car-T cells focused on cancer treatment through immunotherapy. The company was founded in 2016 in Granada as a spin off of the Andalusian Public Health System and the Progreso y Salud Foundation.
The company’s aim is to develop its technologies to generate a more effective and safer Car-T therapy, improving side effects and potency. The company collaborates with the Carlos III Hospital in Madrid and the Hospital Clínic in Barcelona.
Francisco Martín, founder of the company, explains that “we are working on trying to control the way in which the therapeutic gene that is introduced into the target cells is expressed in order to achieve a benefit for the patient.” The research led to three patents with translational possibilities, he said.
In addition, the Car-T strategy or immunotherapy strategy has an efficacy of up to 60% in some types of cancer, such as leukemias or lymphomas, says Martín. This technology continues to gain prominence and during the last two years five treatments have been approved.
The company plans to sell its technology to a large pharmaceutical company in 2023 or 2024
LentiStem is developing two technologies in parallel: AWE, in the middle of the preclinical phase, and Lent-On-Plus, at an earlier stage. The founder points out that AWE is the first system that enables physiological expression of CAR using viral vectors. Lent-On-PLus is the only system on the market that enables doxycycline-inducible expression with clinical application possibilities.
The company is in talks with “a couple of specialized science funds.” “The business model is to do clinical phases I and II and sell our technology to a big pharma,” said LentiStem’s CEO. The company expects that scenario to occur in 2023 or 2024.
LentiStem is based in Granada and has eight people involved in the project. The funds will also be used to expand the staff dedicated entirely to developing the technology, which currently consists of three people. All of the company’s capital is held by the ten founders.
__
(Featured image by madartzgraphics via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in PlantaDoce, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Fintech1 week ago
Fintech1 week agoImpacta VC Backs Quipu to Expand AI-Driven Credit Access in Latin America
-

 Impact Investing7 days ago
Impact Investing7 days agoClimate Losses Drive New Risk Training in Agriculture Led by Cineas and Asnacodi Italia
-
 Biotech2 weeks ago
Biotech2 weeks agoWhy Bioceres Shares Slide Into Penny Stock Territory
-

 Crowdfunding3 days ago
Crowdfunding3 days agoReal Estate Crowdfunding in Mexico: High Returns, Heavy Regulation, and Tax Inequality