Biotech
Significant Phase 3 results for the Belgian-Spanish biotech company Minoryx
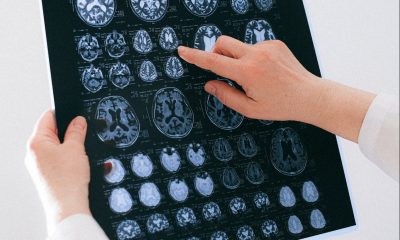
Minoryx has completed a Phase 3 trial with its drug candidate for a neurodegenerative orphan disease and the results are significant. The only downside is that the primary endpoint was not met. It was the evaluation of a change from baseline in a 6-minute walk test. Minoryx hopes that this will not be a barrier to the further process of bringing its treatment to market, given the other important benefits that have been achieved.

The Belgian-Spanish biotech company Minoryx Therapeutics disclosed on Monday, February 15th, the results of its Phase III clinical study to evaluate its drug candidate, leriglitazone, in adrenomyeloneuropathy (AMN), a neurodegenerative orphan disease leading to severe motor disorders that can lead to paralysis.
The primary endpoint for the drug candidate leriglitazone was the observation of a change from baseline in a 6-minute walk test.
Verdict of this clinical trial: many significant improvements in patient outcomes, but one primary endpoint was not met. Conducted with 116 patients, this pivotal multicenter, double-blind, placebo-controlled study showed that leriglitazone “significantly reduced the progression of brain damage and myelopathy-related symptoms,” such as loss of balance, according to Minoryx.
There is an improvement in the quality of life of patients and the improvements seen are greater in patients with more recent disease, said the biotech company based near Barcelona, which has a branch in the Gosselies Biopark.
The only downside is that the primary endpoint was not met. It was the evaluation of a change from baseline in a 6-minute walk test.
Read more details about the Phase 3 clinical trial results for the drug candidate leriglitazone developed by the biotech company Minoryx and find other important breakthroughs in the biotech sector with the born2Invest mobile app. Our companion app covers a wide range of trending topics, from biotech to fintech and cryptocurrencies. Read the latest finance news with Born2Invest and stay on top of the market.
What lessons can be learned from these results?
Simply put, Minoryx hopes that this will not be a barrier to the further process of bringing its treatment to market, given the other important benefits that have been achieved. “If you look at all the data obtained, it is clear that the drug has had a positive effect on these patients and has shown significant clinical benefits in multiple parameters ranging from myelopathy-related symptoms to the progression of brain damage,” said Marc Martinell, Minoryx’s CEO, in L’Echo.
“This specific outcome of the primary endpoint can be easily explained if we consider the date the study was initiated. When we started the trial 4 years ago, the understanding of the data on this disease and its natural history was extremely limited. This is the main reason why the primary endpoint was not met for the whole population,” added the CEO.
A solid foundation
So don’t worry, according to the Minoryx boss. “I don’t think this threatens the future of leriglitazone, quite the contrary. We are convinced that these results will provide a solid basis on which we can build a development path so that leriglitazone can finally be offered to patients. We will discuss this with the regulatory authorities in the near future.”
__
(Featured image by ernestoslava via Pixabay)
DISCLAIMER: This article was written by a third party contributor and does not reflect the opinion of Born2Invest, its management, staff or its associates. Please review our disclaimer for more information.
This article may include forward-looking statements. These forward-looking statements generally are identified by the words “believe,” “project,” “estimate,” “become,” “plan,” “will,” and similar expressions. These forward-looking statements involve known and unknown risks as well as uncertainties, including those discussed in the following cautionary statements and elsewhere in this article and on this site. Although the Company may believe that its expectations are based on reasonable assumptions, the actual results that the Company may achieve may differ materially from any forward-looking statements, which reflect the opinions of the management of the Company only as of the date hereof. Additionally, please make sure to read these important disclosures.
First published in L’Echo, a third-party contributor translated and adapted the article from the original. In case of discrepancy, the original will prevail.
Although we made reasonable efforts to provide accurate translations, some parts may be incorrect. Born2Invest assumes no responsibility for errors, omissions or ambiguities in the translations provided on this website. Any person or entity relying on translated content does so at their own risk. Born2Invest is not responsible for losses caused by such reliance on the accuracy or reliability of translated information. If you wish to report an error or inaccuracy in the translation, we encourage you to contact us.

-

 Fintech5 days ago
Fintech5 days agoMuzinich and Nao Partner to Open Private Credit Fund to Retail Investors
-

 Crowdfunding2 weeks ago
Crowdfunding2 weeks agoSwitzerland’s Crowdfunding Market Remains Stable – Without Growth
-

 Crypto9 hours ago
Crypto9 hours agoBitcoin Traders on DEXs Brace for Downturn Despite Price Rally
-

 Business1 week ago
Business1 week agoDebt-Fueled Markets, Zombie Corporations, and the Coming Reckoning